Table of Contents
The eye development initiates with the interaction between the cells of the lens placode and the presumptive retina structure the eye via a cascade of reciprocal changes that enable the construction of an intricately complex organ.
At gastrulation, the head ectoderm is made competent to respond to the signals of the brain bulges. The involuting prechordal plate and foregut endoderm interact with the adjacent prospective head ectoderm to give the head ectoderm a lens-forming bias.
Eye development: Formation of the eye field- The beginnings of the retina:
Role of Otx2 and Pax6 in the eye development:
- The story begins with the formation of the eye field in the anterior of the neural tube. the anterior portion of the neural tube, where both BMP and the Wnt pathways are inhibited, is specified by Otx2 gene expression.
- Noggin is an important protein, as it not only blocks BMP (Allows Otx2 gene expression), but also prevents expression of the transcription factor ET, one of the first proteins expressed in the eye field.
- However, once Otx2 protein accumulates in the ventral head region, it blocks Noggin’s ability to inhibit the ET gene, and the ET gene is produced.
- one of the genes, regulated by ET is Rx, whose product specify the retina. Rx (for ‘retinal homeobox’) is a transcription factor that acts first by inhibiting Otx2 and second by activating Pax6, the major gene in forming the eye field in the anterior neural plate.
- Pax6 protein is important in specifying the lens and retina.
- Pax6 protein initiates a cascade of transcription factors (such as Six3, Rx, and Sox2) with overlapping functions.
Schematic representation of Dynamic expression of some transcriptions factors:
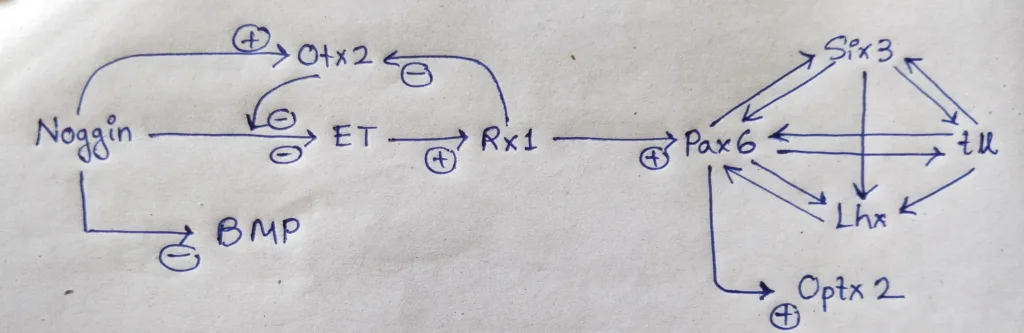
Some mutations cases of Pax6 and Rx:
| Human | Mice | Type of Mutation | Result |
|---|---|---|---|
| Yes | Yes | Heterozygous for loss-of-function mutations in Pax6 | small eyes |
| Yes | Yes | Homozygotic | lack eyes altogether |
| yes | Rx mutant | lack eyes altogether |
Role of Shh in the eye development:
- The cascade of transcription factors initiated by Pax6 mutually activate one another to generate a single eye-forming field in the center of the ventral forebrain.
- The final result is two eyes that are more lateral in the head. The crucial player in separating the single vertebrate eye field into two bilateral fields is Sonic Hedgehog (Shh).
- Shh from the prechordal plate suppresses Pax6 expression in the center of the neural tube, dividing the field in two.
Mutation cases of Shh in the eye development:
| Shh gene | Observation | Result |
|---|---|---|
| mutation | the single median eye field does not split | Cyclopia– a single eye in the center of the face, usually below the nose. |
| overexpression | Pax6 gene is suppressed in too large an area | Eyes fail to form. This phenomenon may explain the cause of blindness of the cave-dwelling fish. |
Eye development: The lens-Retina induction cascade:
- The eye field of the anterior neural plate induces the epidermis above it to become the lens, and each lens begin to form, it induces the eye field to become the retina. The eye development is the example of reciprocal embryonic induction.
- The epidermal tissue has to become competent to respond to the signals sent by the presumptive retinal cells.
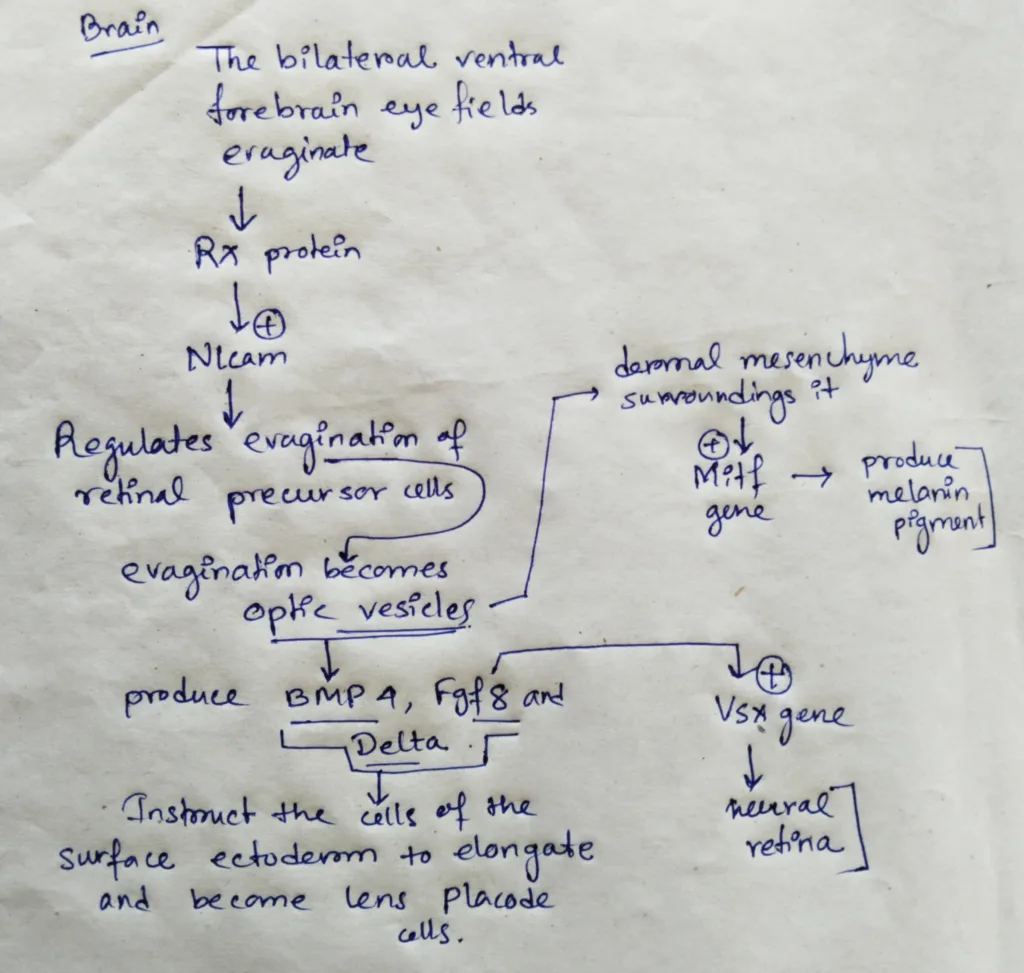
- In the brain, the bilateral ventral fire brain eye fields evaginate as the Rx protein activates Nlcam, a gene whose cell surface product regulates the evagination of the retinal precursor cells from the ventral forebrain.
- The evaginations become the optic vesicles. When the cells of the optic vesicles touch surface ectoderm, both tissues are changed.
- The optic vesicle cells flatten against the surface ectoderm and produce BMP4, Fgf8 and Delta. These inducers instruct the cells of the surface ectoderm to elongate and become lens placode cells. The lens placode cells secrete Fgfs the instruct the adjacent cells of the optic vesicles to activate the Vsx2 gene that characterizes the neural retina.
- The dermal mesenchyme surrounding the optic vesicle instructs most of the outer optic vesicle cells to activate the Mitf gene, which will activate the formation of Melanin pigment.
- Once the eye fields are specified and divided, much of the development into the optic cup is remarkably autonomous.
Schematic representation of the formation of optical vesicle and further development:
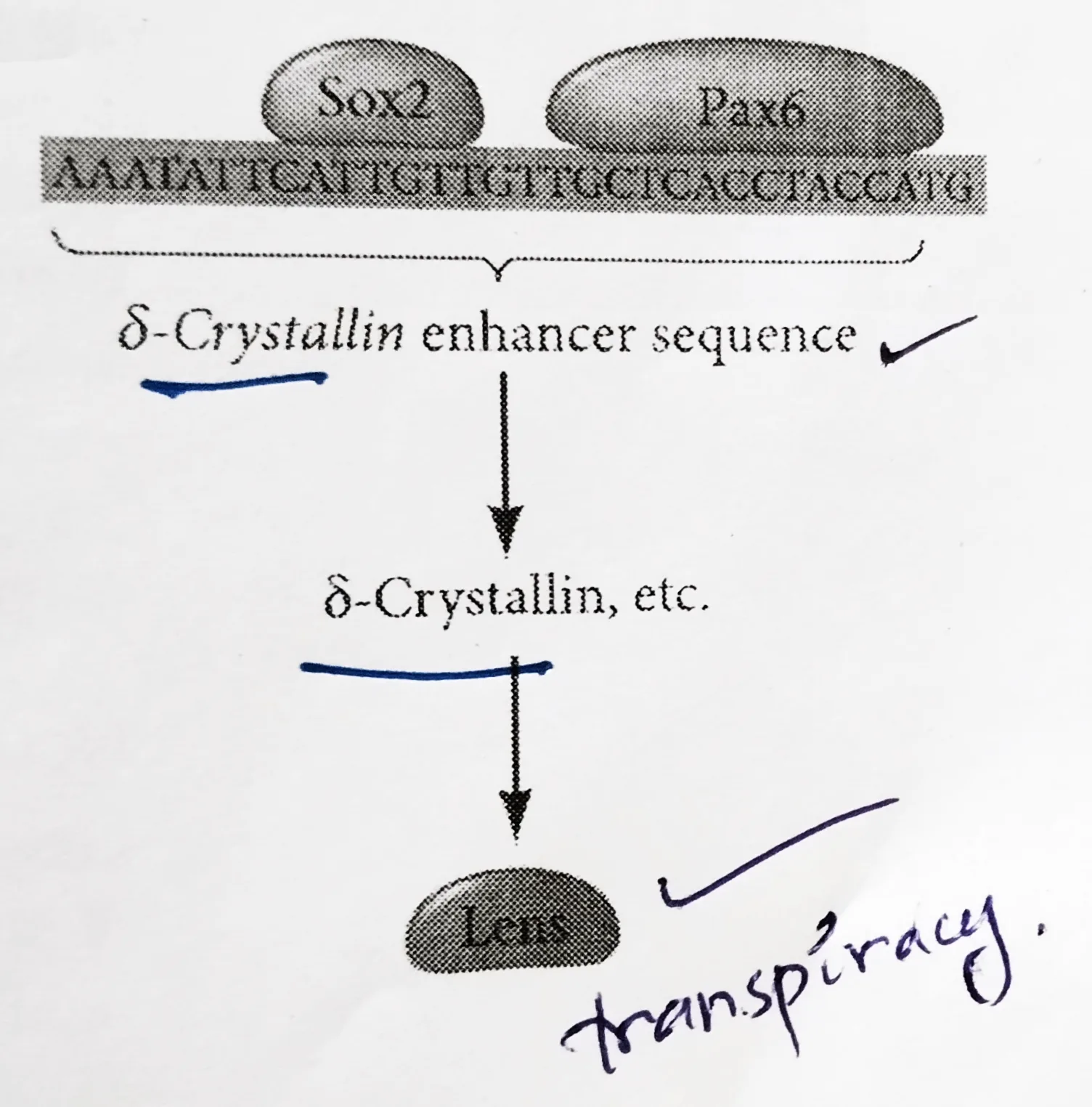
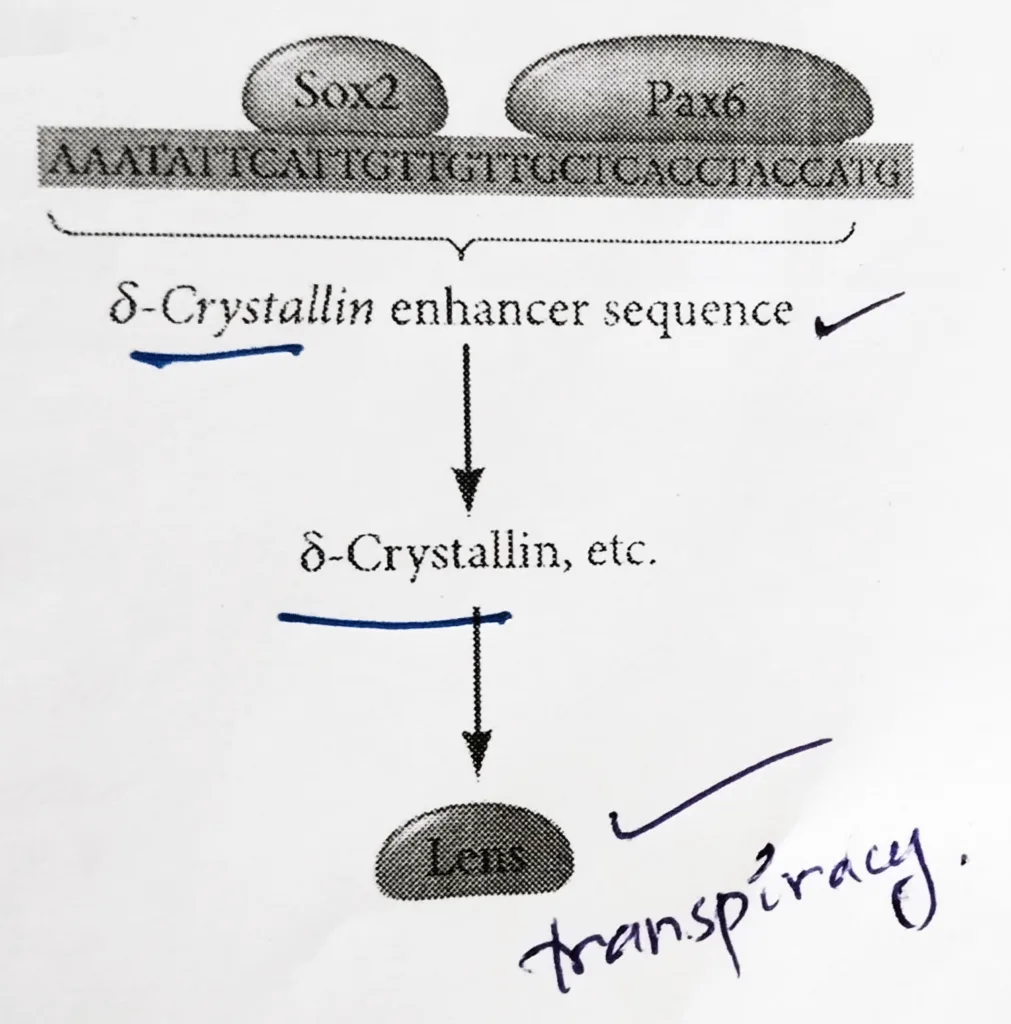
Lens differentiation:
- The differentiation of lens tissue into a transparent membrane capable of directing light onto the retina involves changes in cell structure and shape as well as the synthesis of transparent and lens-specific proteins called crystallins.
- Crystallins account for upto 90% of the lens-soluble proteins.
- The initial differentiation of lens-forming tissues needs contact between the optic vesicle and the presumptive lens ectoderm.
- In addition to preventing neural crest cells from inhibiting the intrinsic lens-specifying bias of the anterior ectoderm, this contact appears to permit Delta proteins on the optic vesicle to activate Notch receptors on the presumptive lens ectoderm.
- The Notch intracellular domain binds to enhancer element of the Lens1 gene. Lens1 gene is activated in the presence of the Otx2 transcription factor.
- Lens1 gene is essential for the growth of lens placode and eventually for closing the lens vesicle.
- Paracrine factors from the optic vesicle also induce the lens-specific transcription factors. Regulation of crystallin genes is under the influence of Pax6, Sox2, and L-Maf.
- Like Otx2, Pax6 appears in the head ectoderm before the lens is formed and Sox2 is induced in the lens placode by BMP4 secreted from the optic vesicle.
- Coexpression of Pax6 and Sox2 in the same cells initiates lens differentiation and activates crystallin genes.
- Appearing later than Sox2, L-Maf are induced by Fgf8 secreted by the optic vesicle and is required for the maintenance of the crystallin gene expression and the completion of lens fiber differentiation.
Schematic representation of the lens differentiation:

Mutation cases of Pax6:
| Optic vesicles | Surface ectoderm | Lens formation |
|---|---|---|
| Wild type | Wild type | Yes |
| Pax6 mutant (Pax6–/Pax6–) | Wild type | Yes |
| Wild type | Pax6 mutant (Pax6–/Pax6–) | No |
| Pax6 mutant (Pax6–/Pax6–) | Pax6 mutant (Pax6–/Pax6–) | No |
Cornea differentiation:
- Shortly after that lens vesicle has detached from the surface ectoderm, the lens vesicle stimulates the overlying ectoderm to become cornea. The molecules necessary for this transformation are probably Dickkopf proteins that inhibit Wnts and Wnt- induced β-catenins.
- In mice, with loss of function mutations in their Dickkopf-2 genes, the corneal epithelium becomes head epidermal tissue.
- The presumptive corneal cells secrete layers of collagen into which neural crest cells migrate and make new cell layers while secreting a corneal-specific extra cellular matrix.
- These cells condense to form several flat layers of cells, becomes corneal precursor cells.
- As these cells mature, they dehydrate and form tight junctions among the cells, uniting with the surface ectoderm to become the cornea.
- The main problem for the cornea is reactive oxygen species (ROS) that damage DNA and proteins. The major source of ROS are the amniotic fluid and ultraviolet light.
- One protective mechanism is the production of the iron& binding protein ferritin.
- The second mode of protection is a layer of basal cells that continually renew the corneal epithelial cells throughout the life of the individual.
- Long-lived stem cells found at the edge of the cornea contribute to corneal repair and can regenerate the cornea in humans. These cells in this region also secrete Dickkopf to prevent their becoming epidermal cells.
The note is made from the book of developmental biology by Gilbert.
Other related notes:
- Gamete fusion and prevention of polyspermy: https://thebiologyislove.com/gamete-fusion-the-prevention-of-polyspermy/
- Patterns of cleavage: https://thebiologyislove.com/patterns-of-cleavage/
- Tetrapod limb development: https://thebiologyislove.com/tetrapod-limbs-development/
Facebook link: https://www.facebook.com/share/p/9zRCsf3zKjrRb9Uu/?mibextid=oFDknk
Instagram link:https://www.instagram.com/p/C6Q0PbSREpE/?igsh=em5pbWYybzJ1bDEx