Table of Contents
Post-translational modifications of proteins is one of the major events after translation (protein formation). There are three major sub-events of post-translational modifications of proteins. The three sub-events are-1) Chemical modification, 2) proteolytic cleavage, and 3) protein splicing.
Post-translational modifications of proteins:- Chemical modification:
Primary translation products often undergo a variety of modification reactions, involving the addition of chemical groups, which are attached covalently to the polypeptide. This can involve simple chemical modification like hydroxylation and phosphorylation of the side chains of single amino acids or the addition of different types of carbohydrate or lipid group.
Some examples of chemical modifications:
| Chemical modifications-Post-translational modifications of proteins | Definition of the chemical modifications | Amino acids that are modified |
|---|---|---|
| Acetylation | In this type of chemical reactions, a hydrogen atom is substituted for an acetyl group in the target compound. | Lys |
| Methylation | In this type of chemical reactions, the methyl group is added to DNA, proteins, or other molecules. | Lys, Arg |
| Phosphorylation | In this type of chemical reactions, the addition of a phosphoryl (PO3) group occurs to a molecule. | Ser, Thr, Tyr, Asp, His, Lys |
| Hydroxylation | In this type of chemical reactions, a hydroxyl (-OH) group is added to an organic compound. | Pro, Lys |
| Carboxylation | In this type of chemical reactions, carboxylic acid is formed by reacting a substrate with carbon-dioxide. | Glu (form γ-carboxyglutamic acid) |
| O-linked glycosylation | In this type of chemical reactions, the attachment of a sugar molecule to the oxygen atom of Ser or Thr residues in a protein happens. | Ser, Thr |
| N-linked glycosylation | In this type of chemical reactions, the attachment of any sugar molecule is happened to a nitrogen atom of Asn residue in a protein. | Asn |
| Acylation | In this type of broad class chemical reactions, an acyl group is added to a substrate. | Ser, Thr, Cys |
| Myristoylation | In this type of chemical reactions, the attachment of 14-carbon fatty acid myristates to the N-terminal glycine of proteins by the enzyme N-myristoyltransferases (NMT) occurs. | Gly |
| Palmitoylation | In this type of chemical reactions, the covalent bond formation of fatty acids occurs. For example, palmitic acid, to cysteine (termed as S-palmitoylation). | Cys |
| Farnesylation | In this type of chemical reactions, by which an isoprenyl group is added (prenylation) to a cysteine residue of a protein. | Cys |
| Biotinylation | In this type of chemical reaction, the biotin is added to proteins. | Lys |
| ADP ribosylation | In this type of chemical reactions, the incorporation of one or more ADP-ribose moieties is occurred to a protein. | At the nitrogen atom of His, Arg, Asn and Lys or at carboxyl group of Glu. |
Post-translational modifications of proteins:- Proteolytic cleavage:
Proteolytic cleavage of polypeptide chains after synthesis is a common occurrence with certain classes of proteins. For example, proteolytic enzymes in the digestive tract are produced in inactive forms that are generally termed as zymogens.
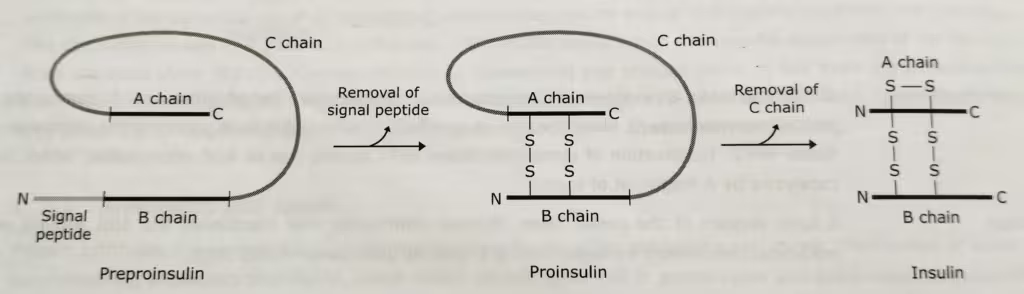
After selective proteolysis, these enzymes are converted into active forms. One famous example of proteolytic cleavage is the conversion of preproinsulin into insulin.
- The removal of the signal peptide from the newly synthesized people, preproinsulin, generates the proinsulin precursor molecule.
- Finally, the removal of C-peptide moiety of proinsulin gives insulin.
The formation of human insulin from preproinsulin:
Post-translational modifications of proteins:- Protein splicing:
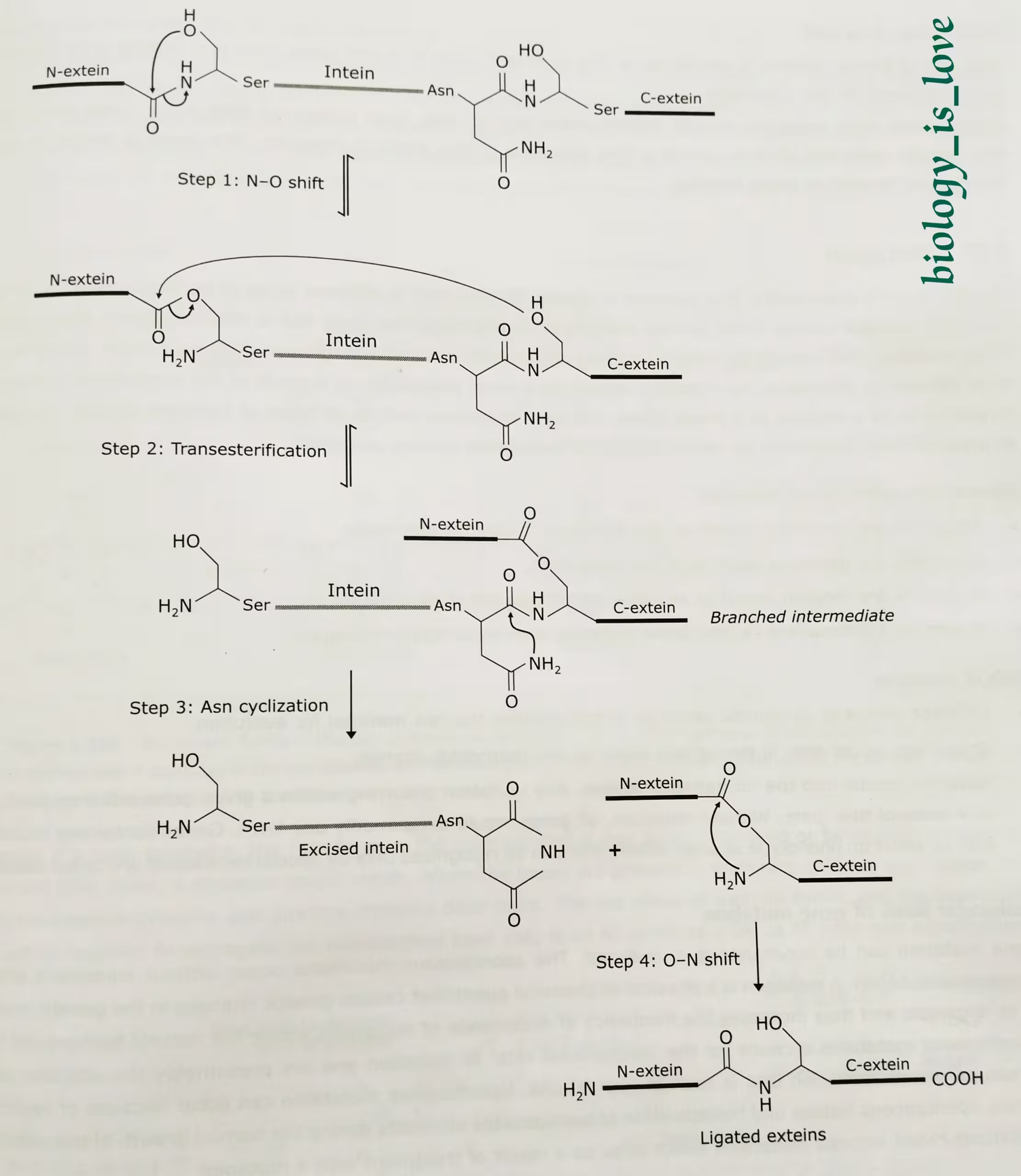
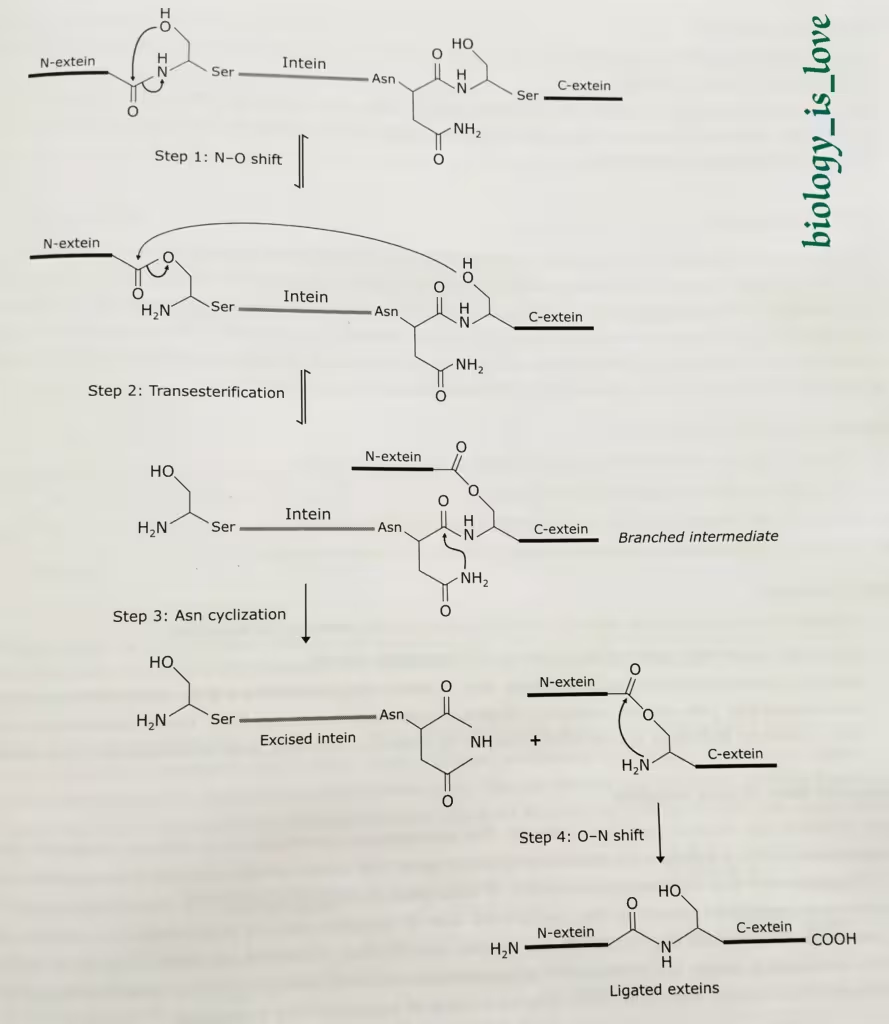
Occasionally, the primary translation product of a gene contains one or more short amino acid sequences, called inteins that excise themselves from the nascent polypeptide. The sequences that are represented in mature polypeptide are termed exteins.
Inteins occur in both eukaryotic and prokaryotic polypeptides. An intein has the ability to catalyze its own removal from primary translation products. The autocatalytic process of protein splicing involves bond transfer reactions and no input of energy is needed. The standard protein splicing mechanism has four steps:
Step-1: N-O shift:
- A typical intein begins with Ser or Cys and end in Asn. The first residue in the C-extein is Ser, Thr or Cys. These residues often function as nucleophiles.
- N-O shift is the transition of the peptide bond between the amino end of the intein and N-extein into an ester or thioester bond.
- This transition depends on a nucleophilic attack of the bond by the side chain of the Ser or Cys residues at the amino terminal end of the intein (-OH or -SH respectively).
- This reaction is termed N-O when the attacking atom is an oxygen and N-S when this atom is sulfur.
Step-2: Transesterification:
- In this phase, the side-chain of the first residue of the first residue of the C-extein attacks the ester (or thioester) bond at the amino end of the intein. Here too the attck is by a polar side chain of a Ser, Thr (both -OH) or Cys (-SH).
- This leads to a transesterification and formation of thioester or ester bond between N-extein and C-extein.
Step-3: Asn cyclization:
- Cyclization of the Asn side chain leads to cleavage of the peptide bond between the intein and the C-extein (C-terminal splice junction).
- This reaction removes intein from the ligated exteins, which are linked together via the ester bond.
Step-4: O-N shift:
- This is the last step of protein splicing.
- This step of protein splicing is spontaneous.
- The reverse N-O or N-S shift takes place and peptide bond formation occurs between N- and C-exteins.
The diagrammatic representation of the mechanism of intein-mediated protein splicing:
Protein splicing-Intein Homing:
Some inteins show sequence specific endonuclease activity also. Such inteins cut DNA in the intein-minus gene at a specific point and allow a copy of a DNA sequence coding intein to integrate. This event is similar to intron homing and referred to as intein homing.
Other related notes:
- 5’capping: https://thebiologyislove.com/5-capping/
- Polyadenylation: https://thebiologyislove.com/polyadenylation/
Facebook link: https://www.facebook.com/share/p/jxj61ny6RBQzXZvh/?mibextid=oFDknk
Instagram link: