Table of Contents
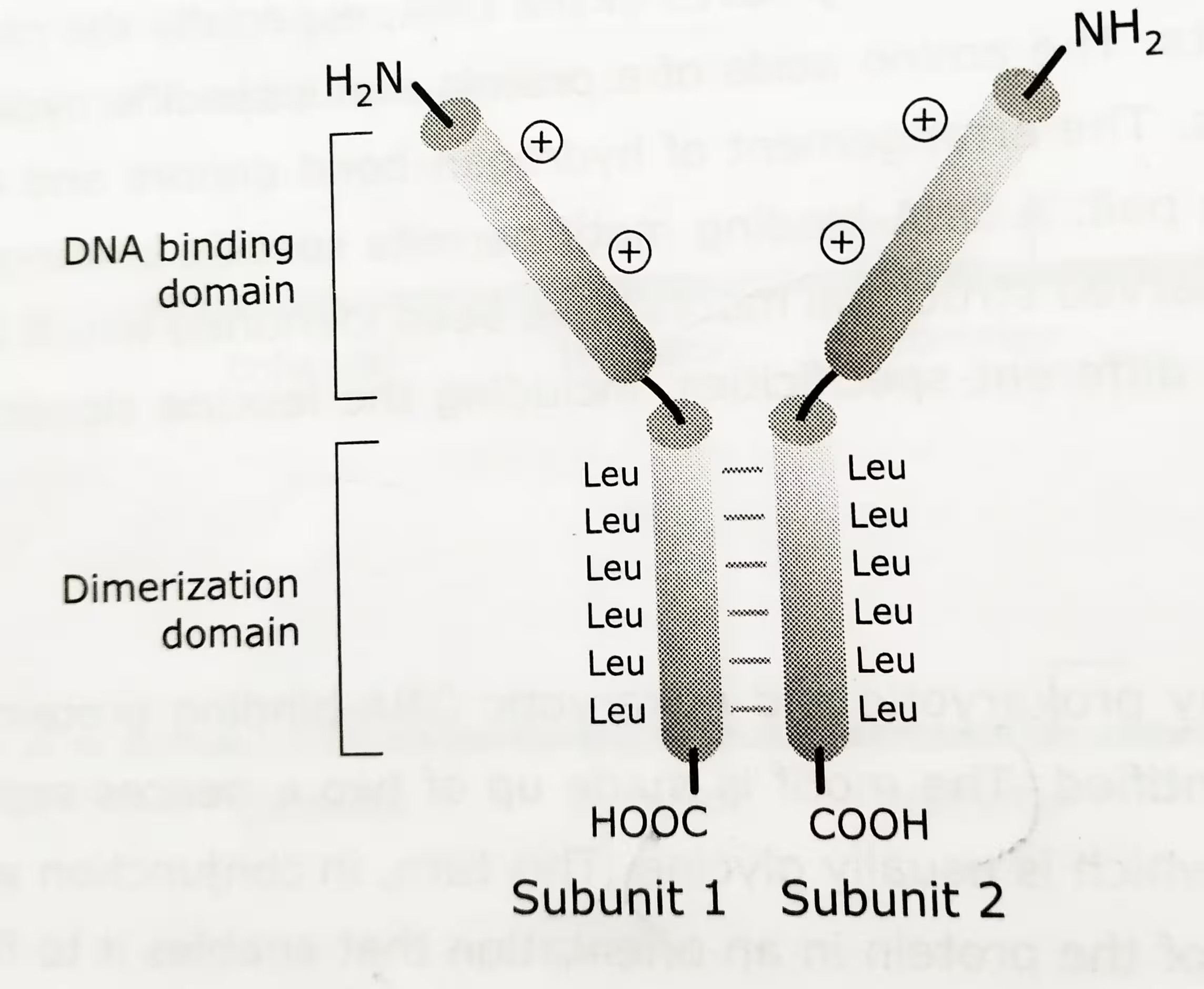
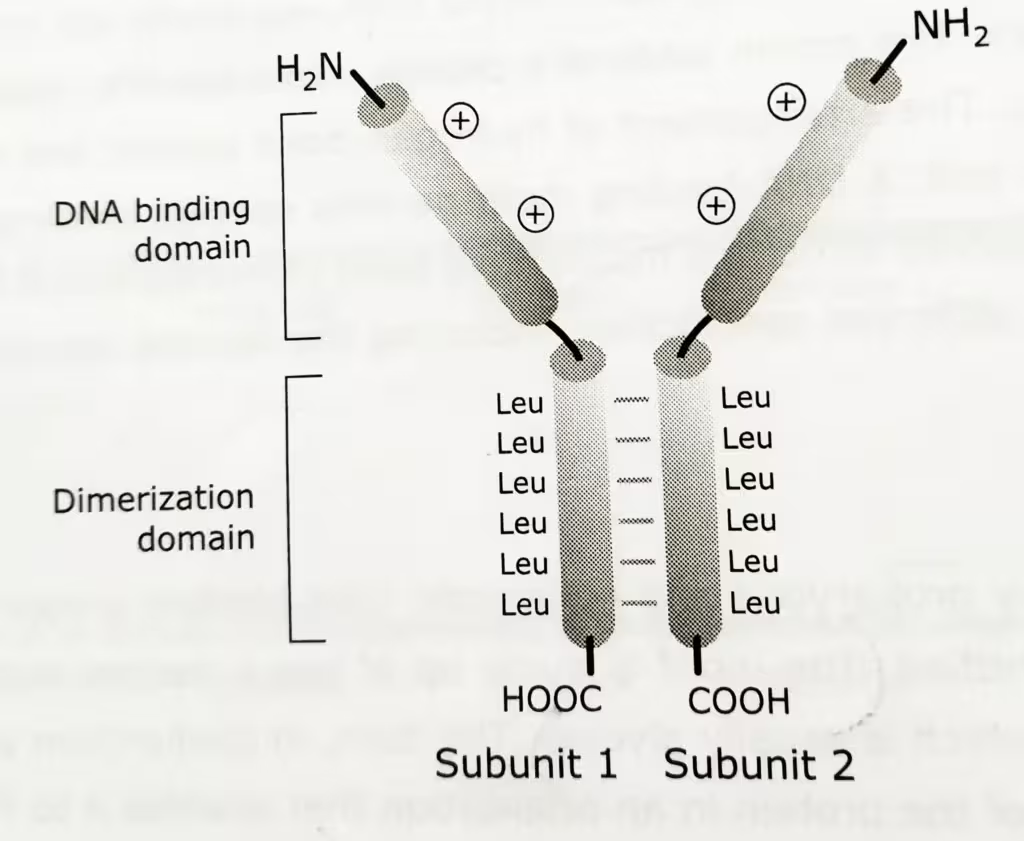
The leucine zipper motif (bZip) mediates both DNA binding and dimerization.
The structure of Leucine Zipper motif:
- Two distinct right handed α helices participate in the formation of homo-or heterodimer structure.
- N-terminal DNA binding domain is rich in positively charged amino acid residues.
- C-terminal dimerization domain is approximately 30-40 amino acids long with a leucine at every seventh position.
- The two α-helices in parallel wind around each other to form a coiled structure.
- The Leucine residues end up with their R-groups protruding from the α helical domain in which the leucine residues reside.
- The protruding R groups are thought to interdigitate with leucine R groups of another leucine zipper domain, thus stabilizing homo- or heterodimerization.
Occurrence:
The Leucine zipper domain is present in many DNA binding proteins, such as c-Myc, Jun, Fos and C/EBP in mammals.
The diagrammatic representation of Leucine-Zipper motif:
Other DNA binding motifs:
- Introduction to DNA binding motifs: https://thebiologyislove.com/dna-binding-motifs/
- helix-turn-helix: https://thebiologyislove.com/helix-turn-helix-motif/
- helix-loop-helix: https://thebiologyislove.com/helix-loop-helix-motif/
- zinc finger motif: https://thebiologyislove.com/zinc-finger-motif-c2-h2/
Facebook link: https://www.facebook.com/share/p/JYvwRUCAydb7oCnw/?mibextid=oFDknk
Instagram link: https://www.instagram.com/reel/C8kMoBvSiSx/?igsh=bWRjZXZkM2JjbTk=