Table of Contents
Each of the adrenal glands has an outer adrenal cortex that secretes the steroid hormones, mineralocorticoids, glucocorticoids and androgens, and an inner medulla that secretes the catecholamines, epinephrine, norepinephrine, and dopamine.
The hormones from adrenal cortex:
- The adrenal cortex secretes glucocorticoids (for example: cortisol) which are steroids with widespread effects on the metabolism of carbohydrate and protein.
- The mineralocorticoid (for example: aldosterone)is essential to the maintenance of Na+ balance and extracellular fluid (ECF) volume.
- Mineralocorticoids and the glucocorticoids are essential for survival.
- It is also a secondary site of androgen synthesis, secreting sex hormones such as testosterone, which can exert effects on reproductive function.
- Adrenocortical secretion is controlled primarily by adrenocorticotropic hormone (ACTH), from the anterior pituitary (adenohypophysis).
- The mineralocorticoid secretion is also matter to independent regulation by circulating factors, of which the most important is angiotensin II, a peptide formed in the bloodstream by the action of renin.
The structure of adrenal cortex:
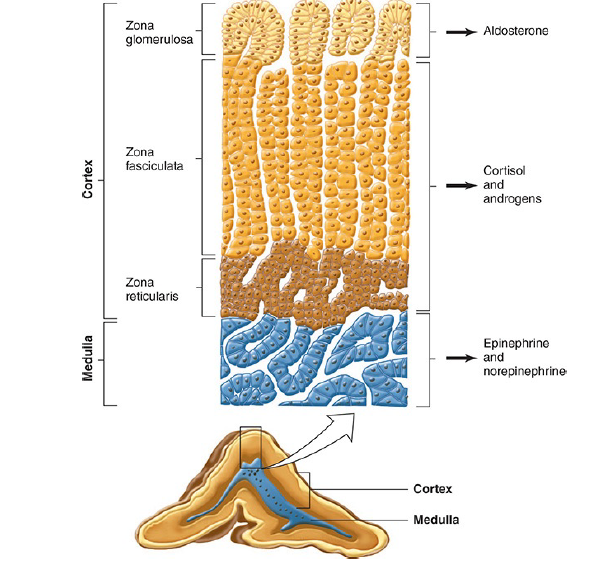
In adult mammals, the adrenal cortex is divided into three regions.
- The outer zona glomerulosa of the adrenal cortex is made up of whorls of cells that are continuous with the columns of cells that form the zona fasciculata. These columns are separated by venous sinuses.
- The middle portion of the zona fasciculata merges into the zona reticularis, (the inner most portion) where the cell columns become interlaced in a network.
- The zona glomerulosa makes up 15% of the mass of the adrenal gland; the zona fasciculata, 50%; and the zona reticularis, 7%.
- The adrenocortical cells contain abundant lipid, especially in the outer portion of the zona fasciculata.
- All three cortical zones secrete corticosterone.
- But the active enzymatic mechanism for aldosterone biosynthesis is limited to the zona glomerulosa.
- The enzymatic mechanisms for forming cortisol and sex hormones are found in the two inner zones.
- Furthermore, sub specialization occurs within the inner two zones, with the zona fasciculata secreting mostly glucocorticoids and the zona reticularis secreting mainly sex hormones.
The blood connection in adrenal cortex
Arterial blood reaches the adrenal from many small branches of the phrenic and renal arteries and the aorta. From a plexus in the capsule, blood flows through the cortex to the sinusoids of the medulla.
The fetal adrenal cortex:
During fetal life, the human adrenal is large and under pituitary control, but the three zones of the permanent cortex represent only 20% of the gland. The remaining 80% is the large fetal adrenal cortex, which undergoes rapid degeneration at the time of birth.
A major function of this fetal adrenal is the synthesizing and secretion of sulfate conjugates of androgens that are converted in the placenta to estrogens. No structure is comparable to the human fetal adrenal in laboratory animals.
Functions:
- In addition to aldosterone synthesis, an important function of the zona glomerulosa is the formation of new cortical cells.
- The adrenal medulla does not regenerate, but when the inner two zones of the cortex are removed, a new zona fasciculata and zona reticularis regenerate from glomerular cells attached to the capsule.
- Small capsular remnants regrow large pieces of adrenocortical tissue.
- Immediately after hypophysectomy, the zona fasciculata and zona reticularis begin to atrophy, whereas the zona glomerulosa is unchanged because of the action of angiotensin II on this zone.
- The ability to secrete aldosterone and conserve Na+ is normal for some time after hypophysectomy,
- But in longstanding hypopituitarism, aldosterone deficiency may develop, apparently because of the absence of a pituitary factor that maintains the responsiveness of the zona glomerulosa.
- Injections of ACTH and stimuli that cause endogenous ACTH secretion produce hypertrophy of the zona fasciculata and zona reticularis but actually decrease, rather than increase, the size of the zona glomerulosa.
- The cells of the adrenal cortex contain large amounts of smooth endoplasmic reticulum (SER), which is involved in the steroid-forming process.
- Other steps in steroid biosynthesis occur in the mitochondria.
- The structure of steroid-secreting cells is very similar throughout the body.
BIOSYNTHESIS HORMONES of the adrenal cortex:
CLASSIFICATION & STRUCTURE
The hormones of the adrenal cortex are derivatives of cholesterol. Like cholesterol, bile acids, vitamin D, and ovarian and testicular steroids, they contain the cyclopentanoperhydrophenanthrene (CPPP) nucleus.
Gonadal and adrenocortical steroids are of three types:
- C21 steroids, which have a two-carbon side chain at position 17;
- C19 steroids, which have a keto or hydroxyl group at position 17;
- C18 steroids, which, in addition to a 17-keto or hydroxyl group, have no angular methyl group attached to position 10.
The adrenal cortex secretes primarily C21 and C19 steroids. Most of the C19 steroids have a keto group at position 17 and are therefore called 17-ketosteroids. The C21 steroids that have a hydroxyl group at the 17 position in addition to the side chain are often called 17-hydroxycorticoids or 17 hydroxy corticosteroids.
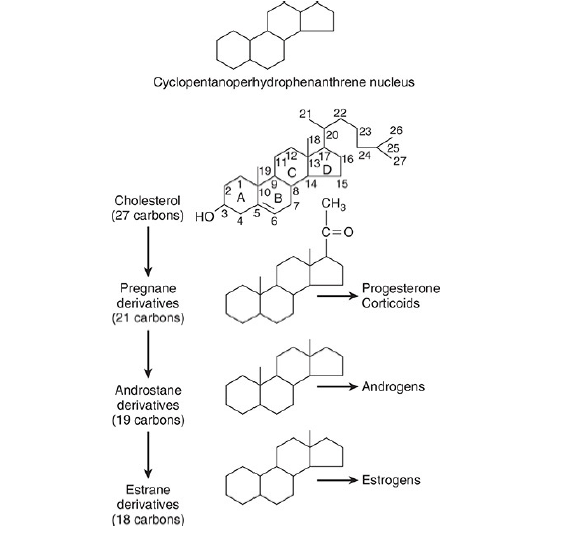
The C19 steroids have androgenic activity. The C21 steroids are classified, using Selye’s terminology, as mineralocorticoids or glucocorticoids. All secreted C21 steroids have both mineralocorticoid and glucocorticoid activity.
Mineralocorticoids are those in which effects on Na+ and K+ excretion predominate and glucocorticoids are those in which effects on glucose and protein metabolism predominate.
The details of steroid nomenclature and isomerism can be found elsewhere. Thus, the C21 steroids secreted by the adrenal have a Δ4-3-keto configuration in the A ring. In most naturally occurring adrenal steroids, 17-hydroxy groups are in the α configuration, whereas 3-, 11-, and 21-hydroxy groups are in the β configuration.
The 18-aldehyde configuration of naturally occurring aldosterone is the D form. L-aldosterone is physiologically inactive.
Adrenal cortex: Secreted steroids:
Innumerable steroids have been isolated from adrenal tissue, but the only steroids normally secreted in physiologically significant amounts are the mineralocorticoid aldosterone, the glucocorticoids cortisol and corticosterone, and the androgens dehydroepiandrosterone (DHEA) and androstenedione.
The structures of these steroids are already shown in the schematic representations below. Deoxycorticosterone is a mineralocorticoid that is normally secreted in about the same amount as aldosterone, but has only 3% of the mineralocorticoid activity of aldosterone.
Its effect on mineral metabolism is usually negligible, but in diseases in which its secretion is increased, its effect can be appreciable. Most of the estrogens that are not formed in the ovaries are produced in the circulation from adrenal androstenedione.
Almost all the dehydroepiandrosterone is secreted conjugated with sulfate, although most if not
all of the other steroids are secreted in the free, unconjugated form.
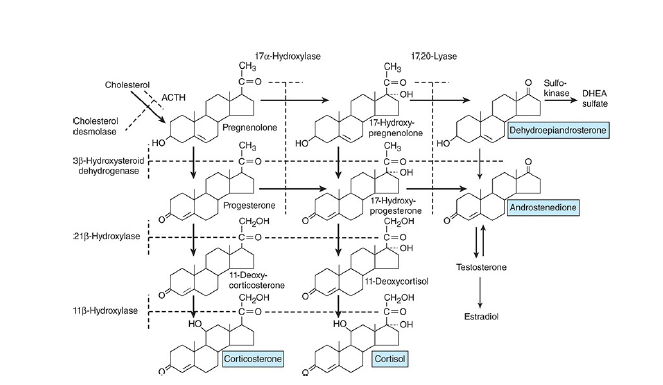
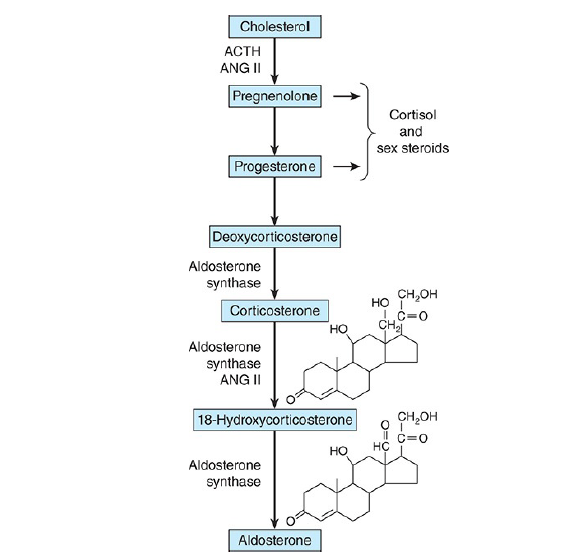
Steroid biosynthesis
The major paths by which the naturally occurring adrenocortical hormones are synthesized in the body are summarized in the above two schematic representations. The precursor of all steroids is cholesterol. Some of the cholesterol is synthesized from acetate, but most of it is taken up from LDL in the circulation.
LDL receptors are especially abundant in adrenocortical cells. The cholesterol is esterified and stored in lipid droplets. Cholesterol ester hydrolase catalyzes the formation of free cholesterol in the lipid droplets (the next figure below).
The cholesterol is transported to mitochondria by a sterol carrier protein. In the mitochondria, it is
converted to pregnenolone in a reaction catalyzed by an enzyme known as cholesterol desmolase or side-chain cleavage enzyme. This enzyme, like most of the enzymes involved in steroid biosynthesis, is a member of the cytochrome P450 superfamily and is also known as P450scc or CYP11A1.
Pregnenolone moves to the smooth endoplasmic reticulum, where some of it is dehydrogenated to form progesterone in a reaction catalyzed by 3β- hydroxysteroid dehydrogenase. This enzyme has a molecular weight of 46,000 and is not a cytochrome P450.
3β- hydroxysteroid dehydrogenase also catalyzes the conversion of 17α- hydroxy pregnenolone to 17α-hydroxyprogesterone, and dehydroepiandrosterone to androstenedione in the smooth endoplasmic reticulum (SER).
The 17α-hydroxy pregnenolone and the 17α-hydroxyprogesterone are formed from
pregnenolone and progesterone, respectively (the above schematic representation) by the action of 17α-
hydroxylase.
This is another mitochondrial P450, and it is also known as P450c17 or CYP17. Located in another part of the same enzyme is 17,20-lyase activity that breaks the 17,20 bond, converting 17α-pregnenolone and 17α-progesterone to the C19 steroids dehydroepiandrosterone and androstenedione.
Hydroxylation of progesterone to 11-deoxycorticosterone and of 17α-hydroxyprogesterone to 11-deoxycortisol occurs in the smooth endoplasmic reticulum (SER). These reactions are catalyzed by 21β-hydroxylase, a cytochrome P450 that is also known as P450c21 or CYP21A2.
11-Deoxycorticosterone and the 11-deoxycortisol move back to the mitochondria, where they are 11-hydroxylated to form corticosterone and cortisol. These reactions occur in the zona fasciculata and zona reticularis and are catalyzed by 11β-hydroxylase, a cytochrome P450 also known as P450c11 or CYP11B1.
In the zona glomerulosa there is no 11β-hydroxylase, but a closely related enzyme called aldosterone synthase is present. This cytochrome P450 is 95% identical to 11β-hydroxylase and is also known as P450c11AS or CYP11B2.
The genes that code CYP11B1 and CYP11B2 are both located on chromosome 8. However, aldosterone synthase is normally found only in the zona glomerulosa. The zona glomerulosa also lacks 17α-hydroxylase. This is why the zona glomerulosa makes aldosterone but fails to make cortisol or sex hormones.
Furthermore, sub specialization occurs within the inner two zones. The zona fasciculata has more 3β-hydroxysteroid dehydrogenase activity than the zona reticularis, and the zona reticularis has more of the cofactors required for the 17,20-lyase activity of 17α-hydroxylase. Therefore, the zona fasciculata makes more cortisol and corticosterone, and the zona reticularis makes more androgens.
Most of the dehydroepiandrosterone that is formed is converted to dehydroepiandrosterone sulfate (DHEAS) by adrenal sulfokinase, and this enzyme is localized in the zona reticularis as well.
Action of ACTH (Adrenocorticotropic hormone) on the adrenal cortex
ACTH (Adrenocorticotropic hormone) binds to high-affinity receptors on the plasma membrane of
adrenocortical cells.
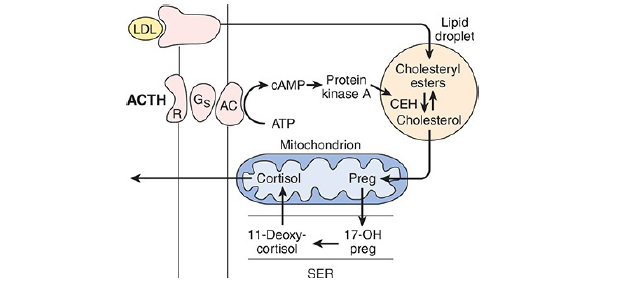
This activates adenylyl cyclase via Gs (G-protein coupled receptor signaling pathway). The resulting
reactions (the above diagram) lead to a prompt increase in the formation of pregnenolone and its derivatives, with secretion of the latter.
Over longer periods, ACTH also increases the synthesis of the P450s involved in the synthesis of glucocorticoids.
Actions of Angiotensin II
Angiotensin II binds to AT1 receptors in the zona glomerulosa of the adrenal cortex that act via a G-protein to activate phospholipase C. The resulting increase in protein kinase C promotes 1) the conversion of cholesterol to pregnenolone and 2) facilitates the action of aldosterone synthase, resulting in increased secretion of aldosterone.
This note is made and the schematic representations are taken from Ganong’s Review of Medical Physiology [26th edition].
Other related notes:
- Dual control of insulin and glucagon: https://thebiologyislove.com/dual-control-insulin-and-glucagon/
- 12th biology important flowcharts: https://thebiologyislove.com/12th-standard-biology-competitive-exam/
Facebook link: https://www.facebook.com/share/p/k3rqDCqQzaVQmpmM/?mibextid=oFDknk
Instagram link:
