
- ASTRAZENECA
Do you know that it is not the name of the vaccine; it is the company sponsored behind the vaccine design. Okay let‟s get into this. The company was founded in 1999 (6th April 1999) through the merger of the Swedish Astra AB (formed in mid 1890s) and the British Zeneca (formed in 1993) Group.
- Current CEO-
Pascal Soriot (1st Oct 2012- present)
- Chairperson-
Leif Johansson
Today, AstraZeneca is working actively over 100 different countries and employs over 57,200 people – 46% in Europe, 31% in the Americas and 23% in Asia-Pacific. Its mission is “to make a meaningful difference to patient health through great medicines that bring benefit for patients and add value for our stakeholders and society”
In 2010 AstraZeneca realized the market need that they should make bold changes to increase „success factor‟ by in-depth R&D result. As a result, they launched the „5R framework‟, which based upon quality over quantity and has transformed the culture of their organisation. Their new model of working solely based upon ensuring each project team focuses on improving the understanding around a key set of criteria to increase the probability of success: right target, right tissue, right safety, right patient and right commercial (see Table 1)
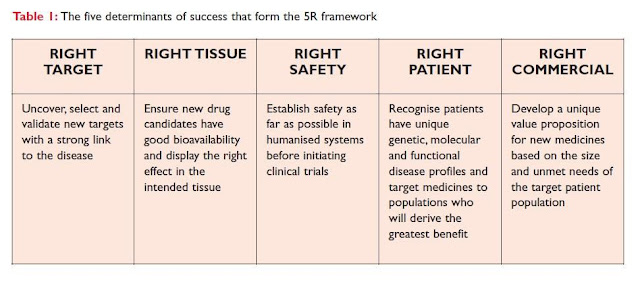
Between 2005 -2010, AstraZeneca‟s preclinical pipeline contained overall 200 projects at any time. After the implementation of this 5R framework, the number of projects halved, with the remaining projects having stronger validation as deemed by the 5R criteria; as the number of projects decreased, the probability of success increased. This quality-over-quantity approach led to decreased clinical attrition, a fuller clinical pipeline and an increase in overall R&D productivity.
Oxford started developing corona virus vaccine in January 2020,
One of key Pioneer behind-
Sara Catherine Gilbert (She is also a key member behind the Ebola virus vaccine).
On 23rd Aril of 2020 the trials began with Dr. Elisa Granato, a 34 year microbiologist injected with the vaccine, and afterwards she told the BBC: “I am a scientist, so I preferred to try to support the scientific method anywhere I can”

A second person was injected with a control vaccine (meningitis vaccine) and both are expected to cause the same mild side effects like any other flu vaccine. Both of them are observed very carefully for 48 hours before six more volunteers got their shots: half with the new vaccine, half with the control.
According to oxford university statement this new vaccine is called ChAdOx1 nCoV-19, and it will also provide “valuable information on safety aspects of the vaccine and its ability to generate good immune responses against the virus”
- Mechanism of action of this vaccine
As per statement it is a recombinant vector vaccine. A live replicating virus (adenovirus) is engineered to carry genes derived from the SARS-CoV-2 virus. Researchers hope that these genes would produce proteins against the virus to generate immunity in the host.
👉ChAdOx1 nCoV-19 is made from a virus (ChAdOx1), which is a weakened version of a common cold virus (adenovirus) that causes infection in chimpanzees that has genetically modified so that it is impossible for it to grow in humans.
👉Genetic material inserted in the ChAdOx1 is used to make proteins from the COVID-19 virus (SARS-CoV-2) called spike-glycoprotein (s). Spike proteins are of two types S1 and S2, these spike proteins on the surface of the COVID-19 virus played a critical role in infection pathway by helping the virus binding in host receptor (ACE-2 receptor).
👉 According to oxford university- “we are hoping to make the body recognise and develop an immune response to the Spike protein that will help the SARS-CoV-2 virus from entering human cells and therefore prevent infection”.
- The control group designed to experience side effects:
The made the groups with all-around 800 participants, half of them randomly received the new vaccine, and a control group received the MenACWY vaccine, a licensed vaccine against group A, C, W and Y meningicoccus which is given routinely to teenagers in the UK. This vaccine mostly protect against common causes of meningitis and sepsis.
- Why this MenACWY??
According to Oxford University they expected to see some minor side effects like sore arm, headache and fever from the ChAdOx1 nCoV-19. Any other salient control does not cause any of these side effects. Their target is to observe if participants were to receive only this vaccine or a saline control, and went to develop side effects, they would be aware that they had received the new vaccine. But as per oxford it was critical for the study that participants were remain blind to whether or not they have received the vaccine, as if they knew, this could affect their health behaviour in the community following vaccination, and this would lead to bias in the results of the study.
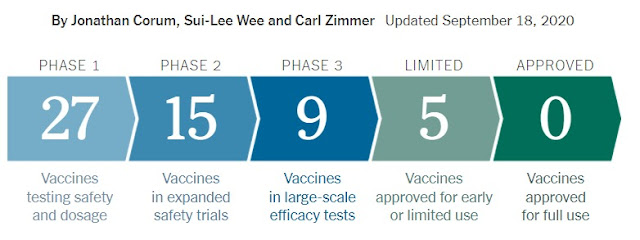
Vaccines typically require years of research and testing before reaching the clinic, for example BCG vaccine requires 13 years for preparation. but our scientists are competing against time to produce a safe and effective coronavirus vaccine within the next year. There are almost 135 vaccines are in trial and 40 of them are in clinical trials on humans and at least 92 preclinical vaccines are under active investigation in animals.
After completing all phase successfully AstraZeneca‟s ChAdOx-1 is in third trial phase and in India Oxford University is collaborated with Serum Institute of India so that they can prepare the vaccine in lower price which could be very helpful for poor (economically) countries, & according to Serum institute of India they can probably release the vaccine at the end of 2020. So, hope that we will win this fight very soon and rise again as before, till then we should maintain proper precautions to remain safe and healthy.
- References
1. https://www.ox.ac.uk/news/2020-04-23-oxford-covid-19-vaccine-begins-human-trial-stage
2. https://health.economictimes.indiatimes.com/news/pharma/good-news-on-oxford-astrazenecas-coronavirus-vaccine-could-come-on-thursday-report/76987945
3. https://thenewdaily.com.au/life/wellbeing/2020/04/24/coronavirus-vaccine-september-oxford/
4. BBC NEWS

