Table of Contents
Catalytic efficiency is also called the specific constant.
Introduction:
An enzyme with a high Km requires a higher substrate concentration to achieve a given reaction velocity than an enzyme with a low Km for the same Kcat (catalytic constant or turnover number). At very low substrate concentrations, where [S] << Km, most of the enzyme is free.
Thus, it is matter to think that [E]=[Et], where Et is the total concentration of enzyme. So that the Michaelis Menten equation becomes in another way, which is presented in the next segment.
The equation:
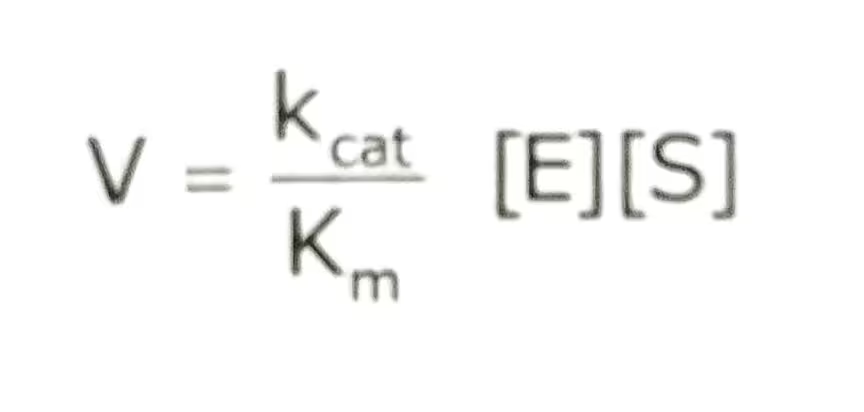
The description of the Catalytic efficiency:
Thus, the ratio Kcat/Km is equivalent to the rate constant for the reaction between the free enzyme and the free substrate. Kcat/Km is the ratio called specific constant is a measure of catalytic efficiency. It means how efficiently an enzyme converts substrate to product at low substrate concentration. It has units of M-1s-1.
Thus, the rate of product formation is dependent on both how well the enzyme binds substrate and how fast the enzyme converts the substrate into a product once the substrate is bound.
Note that, either a large value of Kcat (which means high turnover number of an enzyme) or a small value of Km (which means high affinity for the substrate) will make Kcat/Km large.
A comparison of Kcat/Km for the same enzyme with different substrates, or for two enzymes with with different substrates, is widely used as a measure of Catalytic efficiency.
Other related notes:
Enzyme inhibition: https://thebiologyislove.com/enzyme-inhibition/
Facebook link: https://www.facebook.com/share/p/bpmfzrcykbpQzq3S/?mibextid=oFDknk
Instagram link:
