Table of Contents
Each adrenal gland has an outer cortex that secretes the steroid hormones, mineralocorticoids, glucocorticoids and androgens, and an inner adrenal medulla that secretes the catecholamines,
epinephrine, norepinephrine, and dopamine
Blood connection in the adrenal medulla
From a plexus in the capsule, blood flows through the cortex to the sinusoids of the medulla. The medulla is also supplied by a few arterioles that pass directly to it from the capsule. In most species, including humans, blood from the medulla flows into a central adrenal vein. The blood flow through the adrenal is large, as it is in most endocrine glands.
Adrenal medulla: structure and functions of medullary hormones:
Norepinephrine, epinephrine, and small amounts of dopamine are synthesized by
the adrenal medulla. These two hormones -Norepinephrine, epinephrine are called catecholamines together.
CATECHOLAMINES
Cats and some other species secrete mainly norepinephrine, but in dogs and humans, most of the catecholamine output in the adrenal vein is epinephrine. Norepinephrine also enters the circulation from noradrenergic nerve endings.
Norepinephrine is formed by hydroxylation and decarboxylation of tyrosine. Epinephrine is produced by methylation of norepinephrine. Phenylethanolamine- Nmethyltransferase (PNMT), the enzyme that catalyzes the formation of epinephrine from norepinephrine.
A] The relation of glucocorticoid in the formation of catecholamines:
- This Phenylethanolamine- Nmethyltransferase (PNMT) enzyme is found in appreciable quantities only in the brain and the adrenal medulla. Adrenal medullary PNMT is induced by glucocorticoids. Although relatively large amounts are required, the glucocorticoid concentration is high in the blood draining from the cortex to the medulla.
- After hypophysectomy, the glucocorticoid concentration of this blood falls and epinephrine synthesis is decreased.
- In addition, glucocorticoids are apparently necessary for the normal development of the adrenal medulla.
- In 21β- hydroxylase deficiency, glucocorticoid secretion is reduced during fetal life and the adrenal medulla is dysplastic.
- In untreated 21β-hydroxylase deficiency, circulating catecholamines are low after birth.
B] Comparative study between catecholamines:
- In plasma, about 95% of the dopamine and 70% of the norepinephrine and epinephrine are conjugated to sulfate.
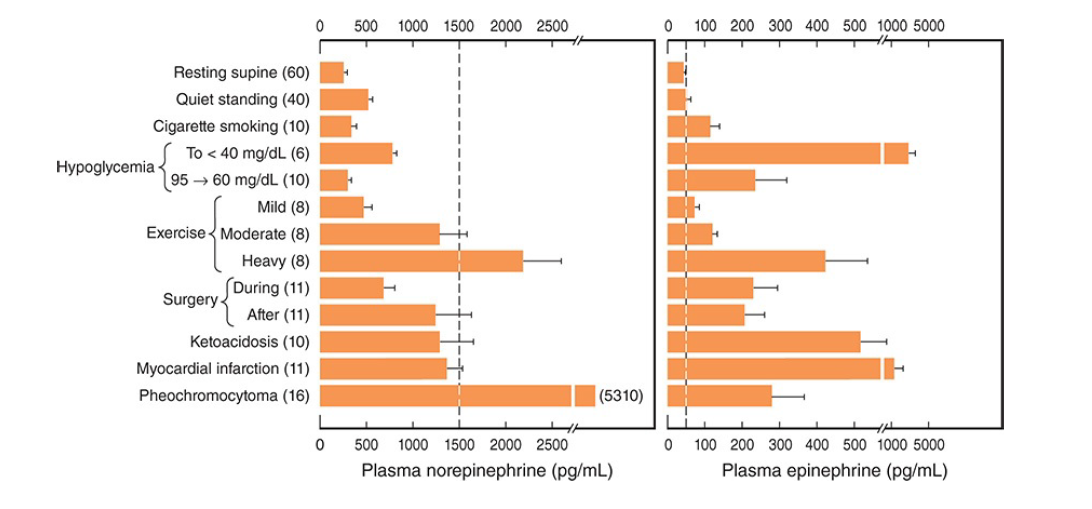
- Sulfate conjugates are inactive and their function is unsettled. In recumbent humans, the normal plasma level of free norepinephrine is about 300 pg/mL (1.8 nmol/L).
- On standing, the level increases 50–100% (Figure 19–4).
- The plasma norepinephrine level is generally unchanged after adrenalectomy, but the free epinephrine level, which is normally about 30 pg/mL (0.16 nmol/L), falls to essentially zero.
- The epinephrine found in tissues other than the adrenal medulla and the brain is for the most part absorbed from the bloodstream rather than synthesized in situ.
- Interestingly, low levels of epinephrine reappear in the blood some time after bilateral adrenalectomy, and these levels are controlled like those secreted by the adrenal medulla.
- They may come from cells such as the intrinsic cardiac adrenergic (ICA) cells, but their exact source is unknown.
Plasma dopamine levels are normally very low, about 0.13 nmol/L. Most plasma dopamine is thought to be derived from sympathetic noradrenergic ganglia.
The catecholamines have a half-life of about 2 min in the circulation. For the most part, they are methoxylated and then oxidized to 3-methoxy-4- hydroxymandelic acid (vanillylmandelic acid [VMA].
About 50% of the secreted catecholamines appear in the urine as free or conjugated metanephrine and normetanephrine, and 35% as VMA. Only small amounts of free norepinephrine and epinephrine are excreted. In normal humans, about 30 μg of norepinephrine, 6 μg of epinephrine, and 700 μg of VMA are excreted per day.
C] Other substances secreted from the adrenal medulla
In the medulla, norepinephrine and epinephrine are stored in granules with ATP. The granules also contain chromogranin A. Secretion is initiated by acetylcholine released from the preganglionic neurons that innervate the secretory cells.
Acetylcholine activates cation channels allowing Ca2+ to enter the cells from the ECF and trigger the exocytosis of the granules. In this fashion, catecholamines, ATP, and proteins from the granules are all released into the blood together.
Epinephrine-containing cells of the medulla also contain and secrete opioid peptides. The precursor molecule is pre proenkephalin. Most of the circulating metenkephalin comes from the adrenal medulla. The circulating opioid peptides do not cross the blood-brain barrier.
Adrenomedullin, a vasodepressor polypeptide found in the adrenal medulla.
Effects of epinephrine and norepinephrine
- In addition to mimicking the effects of noradrenergic nervous discharge, norepinephrine and epinephrine exert metabolic effects that include glycogenolysis in liver and skeletal muscle, mobilization of free fatty acids (FFA), increased plasma lactate, and stimulation of the metabolic rate.
- The effects of norepinephrine and epinephrine are brought about by actions on two classes of receptors: α- and β-adrenergic receptors.
- α-Receptors are subdivided into two groups, α1 and α2 receptors,
- β-receptors into three groups, β1, β2, and β3 receptors.
- There are three subtypes of α1-receptors and
- three subtypes of α2-receptors.
- Norepinephrine and epinephrine both increase the force and rate of contraction of the isolated heart. These responses are mediated by β1-receptors.
- The catecholamines also increase myocardial excitability, causing extrasystoles and, occasionally, more serious cardiac arrhythmias.
- Norepinephrine produces vasoconstriction in most if not all organs via α1-receptors,
- but epinephrine dilates the blood vessels in skeletal muscle and the liver via β2-receptors.
- This usually overbalances the vasoconstriction produced by epinephrine elsewhere, and the total peripheral resistance drops.
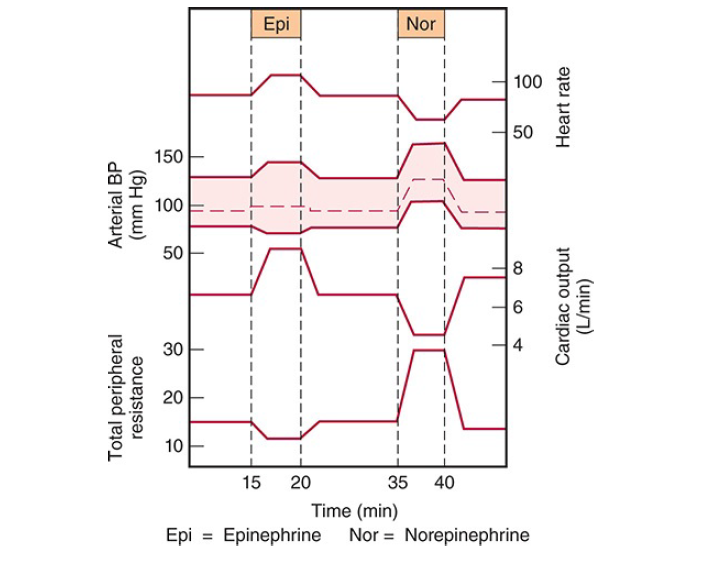
- When norepinephrine is infused slowly in normal animals or humans, the systolic and diastolic blood pressures rise.
- The hypertension stimulates the carotid and aortic baroreceptors, producing reflex bradycardia that overrides the direct cardio acceleratory effect of norepinephrine.
- Consequently, cardiac output per minute falls.
- Epinephrine causes a widening of the pulse pressure but because baroreceptor stimulation is insufficient to obscure the direct effect of the hormone on the heart, cardiac rate and output increase.
- These changes are summarized in the diagram below.
Catecholamines increase alertness: [Some experimental evidences]
- Epinephrine and norepinephrine are equally potent in this regard, although in humans epinephrine
usually evokes more anxiety and fear. - The catecholamines have several different actions that affect blood glucose.
- Epinephrine and norepinephrine both cause glycogenolysis.
- They produce this effect via β-adrenergic receptors that increase cyclic adenosine monophosphate
(cAMP), with activation of phosphorylase, and via α-adrenergic receptors that increase intracellular Ca2+. - In addition, the catecholamines increase the secretion of insulin and glucagon via β-adrenergic mechanisms and inhibit the secretion of these hormones via α-adrenergic mechanisms.
- Norepinephrine and epinephrine also produce a prompt rise in the metabolic rate that is independent of the liver and a smaller, delayed rise that is abolished by hepatectomy and coincides with the rise in blood lactate concentration.
- The initial rise in metabolic rate may be due to cutaneous vasoconstriction, which decreases heat loss and leads to a rise in body temperature, or to increased muscular activity, or both.
- The second rise is probably due to oxidation of lactate in the liver.
- Mice unable to make norepinephrine or epinephrine because their dopamine β-hydroxylase gene is knocked out are intolerant of cold, but surprisingly, their basal metabolic rate is elevated. The cause of this elevation is unknown.
- When injected, epinephrine and norepinephrine cause an initial rise in plasma K+ because of release of K+ from the liver and then a prolonged fall in plasma K+ because of an increased entry of K+ into skeletal muscle that is mediated by β2-adrenergic receptors.
- Some evidence suggests that activation of α-receptors opposes this effect.
- The increases in plasma norepinephrine and epinephrine that are needed to produce the various effects listed above have been determined by infusion of catecholamines in resting humans.
- In general, the threshold for the cardiovascular and the metabolic effects of norepinephrine is about 1500 pg/mL, that is, about five times the resting value.
- Epinephrine, on the other hand, produces tachycardia when the plasma level is about 50 pg/mL, that
is, about twice the resting value. - The threshold for increased systolic blood pressure and lipolysis is about 75 pg/mL; the threshold for hyperglycemia, increased plasma lactate, and decreased diastolic blood pressure is about 150
pg/mL; and the threshold for the α-mediated decrease in insulin secretion is about 400 pg/mL. - Plasma epinephrine often exceeds these thresholds.
- On the other hand, plasma norepinephrine rarely exceeds the threshold for its cardiovascular and metabolic effects, and most of its effects are due to its local release from postganglionic sympathetic neurons.
- Most adrenal medullary tumors (pheochromocytomas) secrete norepinephrine or epinephrine or both and produce sustained hypertension.
- However, 15% of epinephrine-secreting tumors secrete this catecholamine episodically, producing intermittent bouts of palpitations, headache, glycosuria, and extreme systolic hypertension.
- These same symptoms are produced by intravenous injection of a large dose of
epinephrine.
Effects of Dopamine
- The physiologic function of the dopamine in the circulation is unknown.
- However, injected dopamine produces renal vasodilation, probably by acting on a specific dopaminergic receptor.
- It also produces vasodilation in the mesentery.
- It produces vasoconstriction, probably by releasing norepinephrine, and it has a positive inotropic effect on the heart by an action on β1-adrenergic receptors.
- The net effect of moderate doses of dopamine is an increase in systolic pressure and no change in diastolic pressure. Because of these actions, dopamine is useful in the treatment of traumatic and cardiogenic shock.
- Dopamine is made in the renal cortex. It causes natriuresis and may exert this effect by inhibiting renal Na, K, ATPase.
The regulation of the secretion from adrenal medulla
Neural control
Certain drugs act directly on the adrenal medulla, but physiologic stimuli affect medullary secretion through the nervous system. Catecholamine secretion is low in basal states, but the secretion of epinephrine and, to a lesser extent, that of norepinephrine is reduced even further during sleep.
Increased adrenal medullary secretion is part of the diffuse sympathetic discharge provoked in emergency situations, which Cannon called the “emergency function of the sympathoadrenal system.” The ways in which this discharge prepares the individual for flight or fight and the increases in plasma catecholamines under various conditions are shown in previous diagram.
The metabolic effects of circulating catecholamines are probably important, especially in certain situations. The calorigenic action of catecholamines in animals exposed to cold is an example, and so is the glycogenolytic effect in combating hypoglycemia.
Selective secretion
When adrenal medullary secretion is increased, the ratio of norepinephrine to epinephrine in the adrenal effluent is generally unchanged. However, norepinephrine secretion tends to be selectively increased by emotional stresses with which the individual is familiar, whereas epinephrine secretion rises selectively in situations in which the individual does not know what to expect.
This note is made and the schematic representations are taken from Ganong’s Review of Medical Physiology [26th edition].
Other related notes:
- Adrenal cortex: https://thebiologyislove.com/adrenal-cortex/
- 12th Biology important flowcharts: https://thebiologyislove.com/12th-standard-biology-competitive-exam/
Facebook link: https://www.facebook.com/share/p/GizhPZAXpKa9nDig/?mibextid=oFDknk
Instagram link: